Building synergies, one cause at a time. The Synergist 2018 report
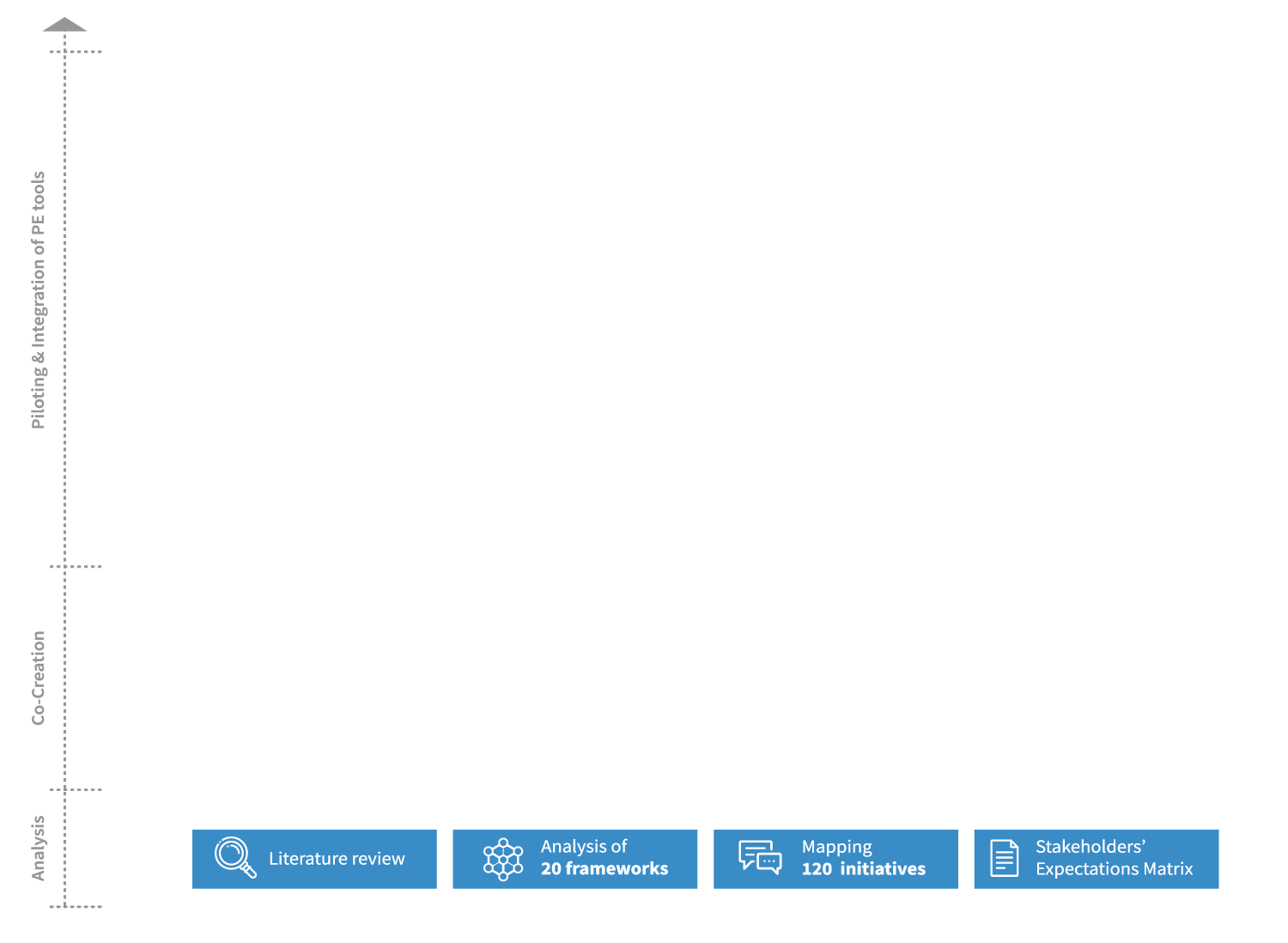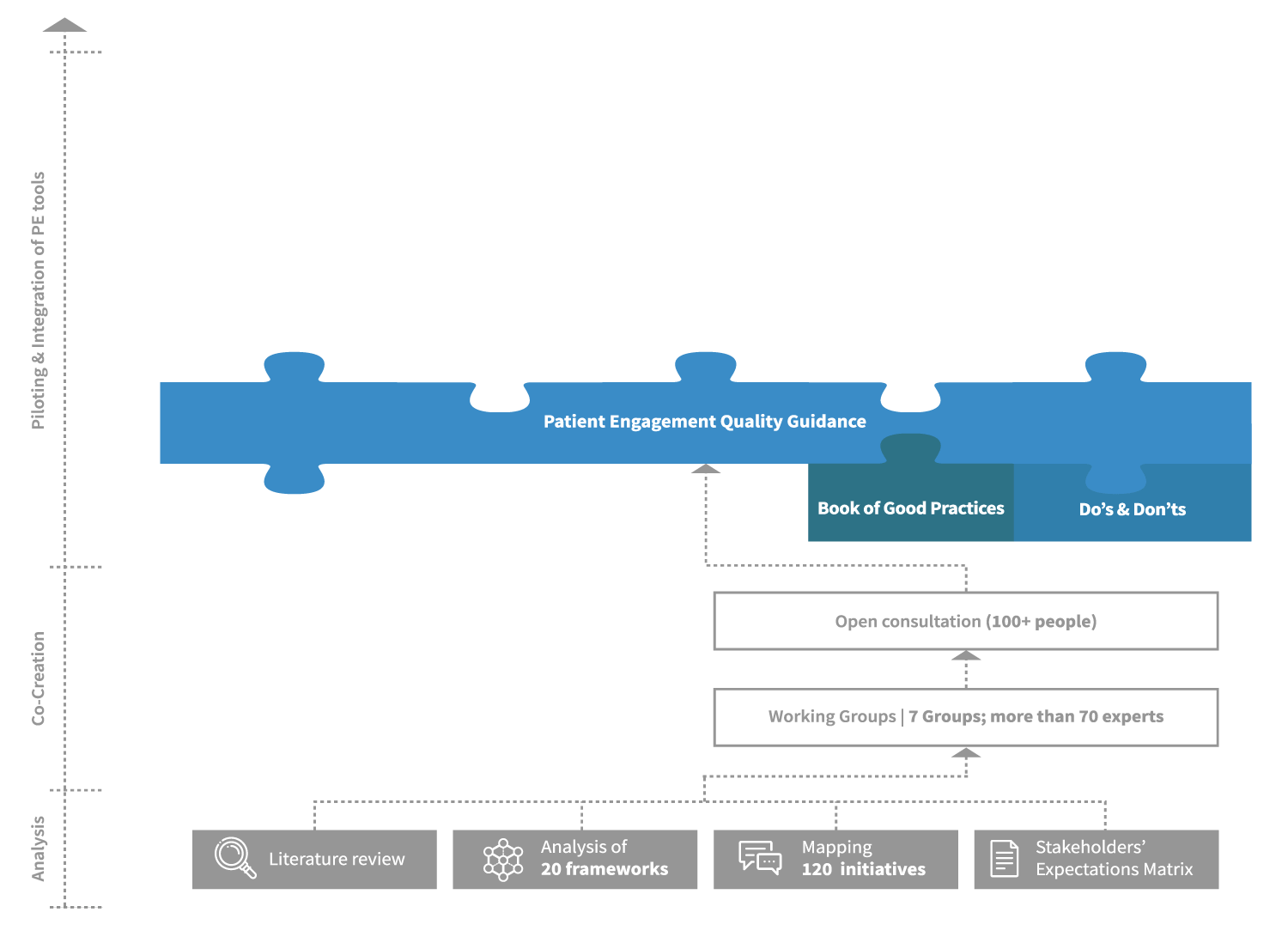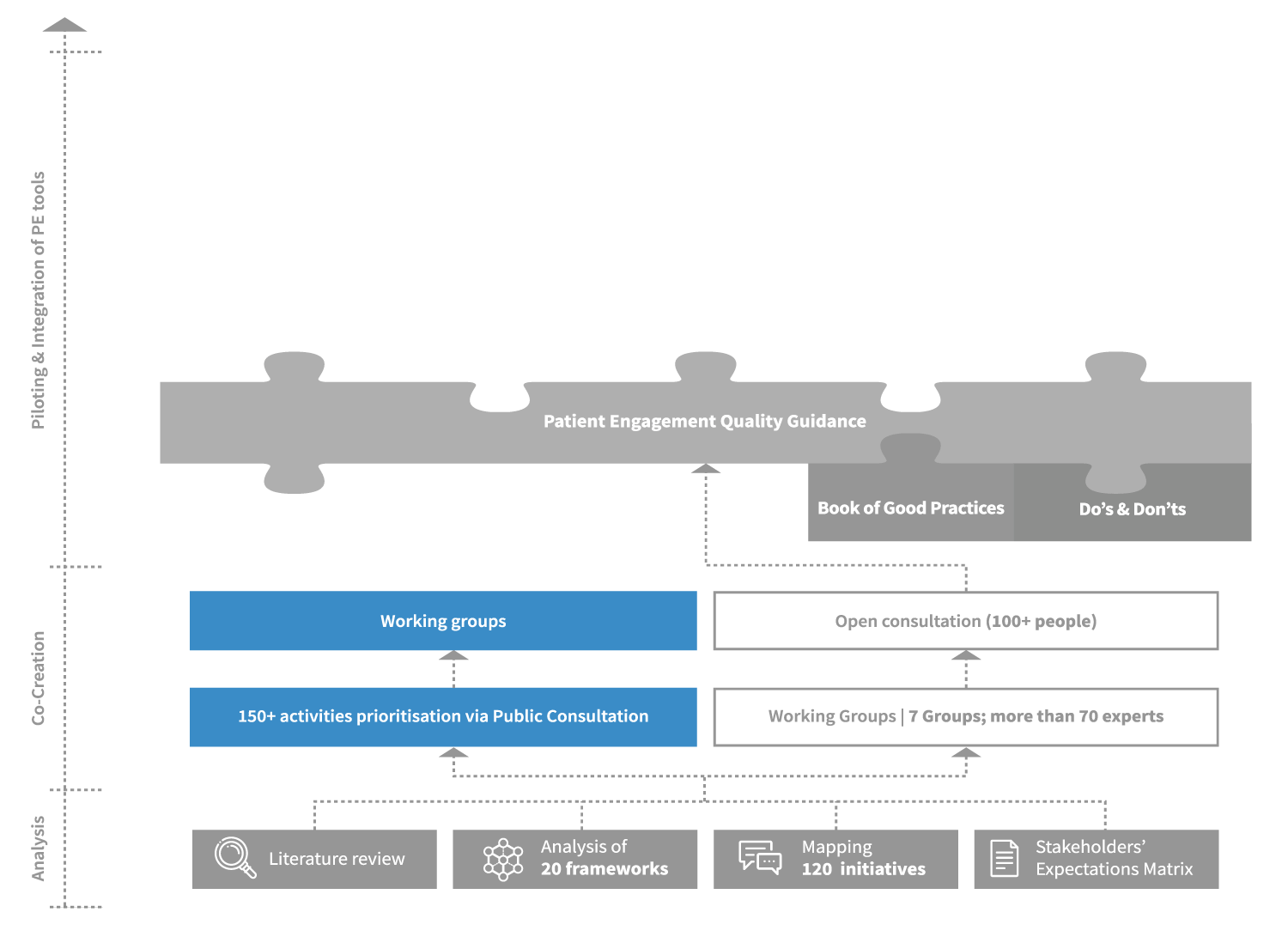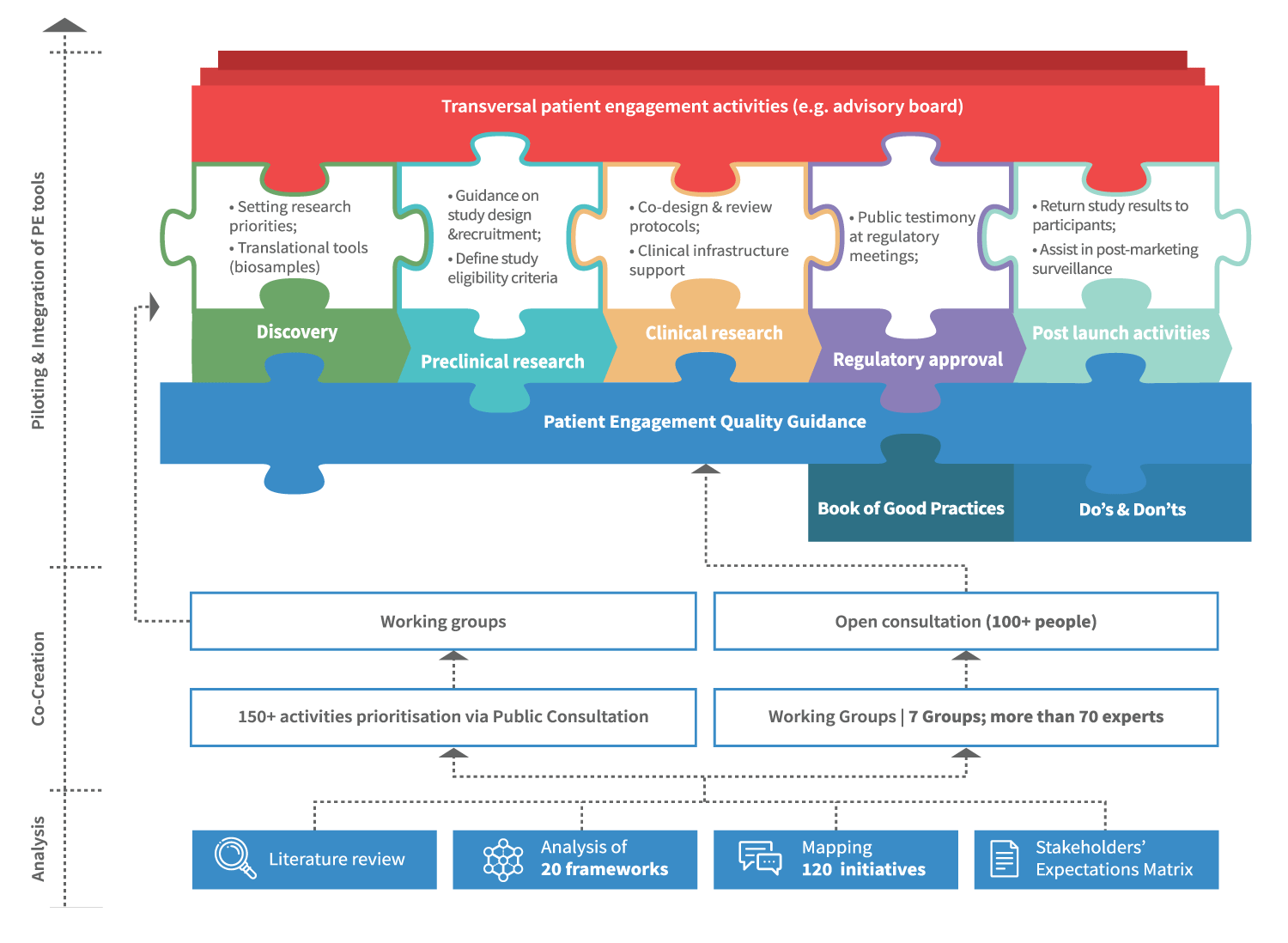
The Patient Engagement Quality Guidance
We are now witnessing a paradigm shift in patient engagement. Instead of contemplating the risk of doing patient engagement, growing numbers of decision-makers in medicines development are speaking of the risks of not doing patient engagement.
The co-developed Patient Engagement Quality Guidance has been embraced by the patient engagement community which has shared it internally and beyond and utilised it for patient engagement projects.
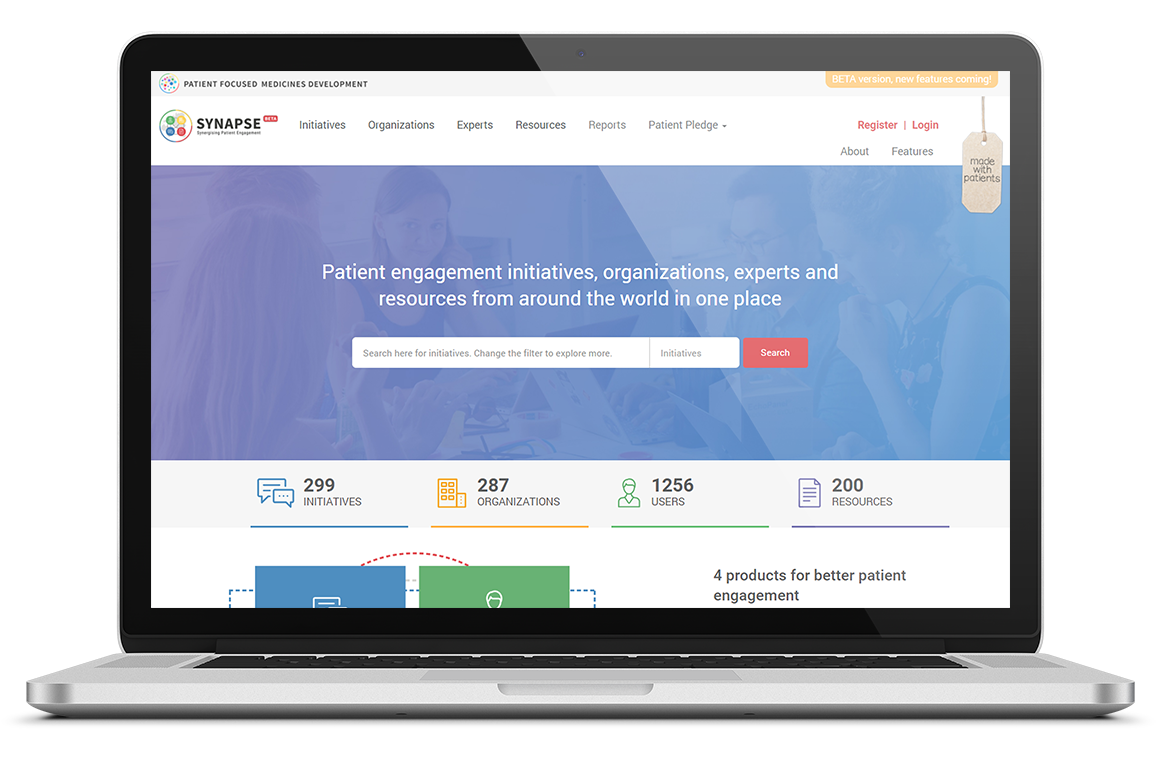
Read moreThe SYNaPsE platform
In 2018, the SYNaPsE platform has evolved from mapping Patient Engagement initiatives to also connecting the whole Patient Engagement ecosystem of experts and their organisations to initiatives and related resources.
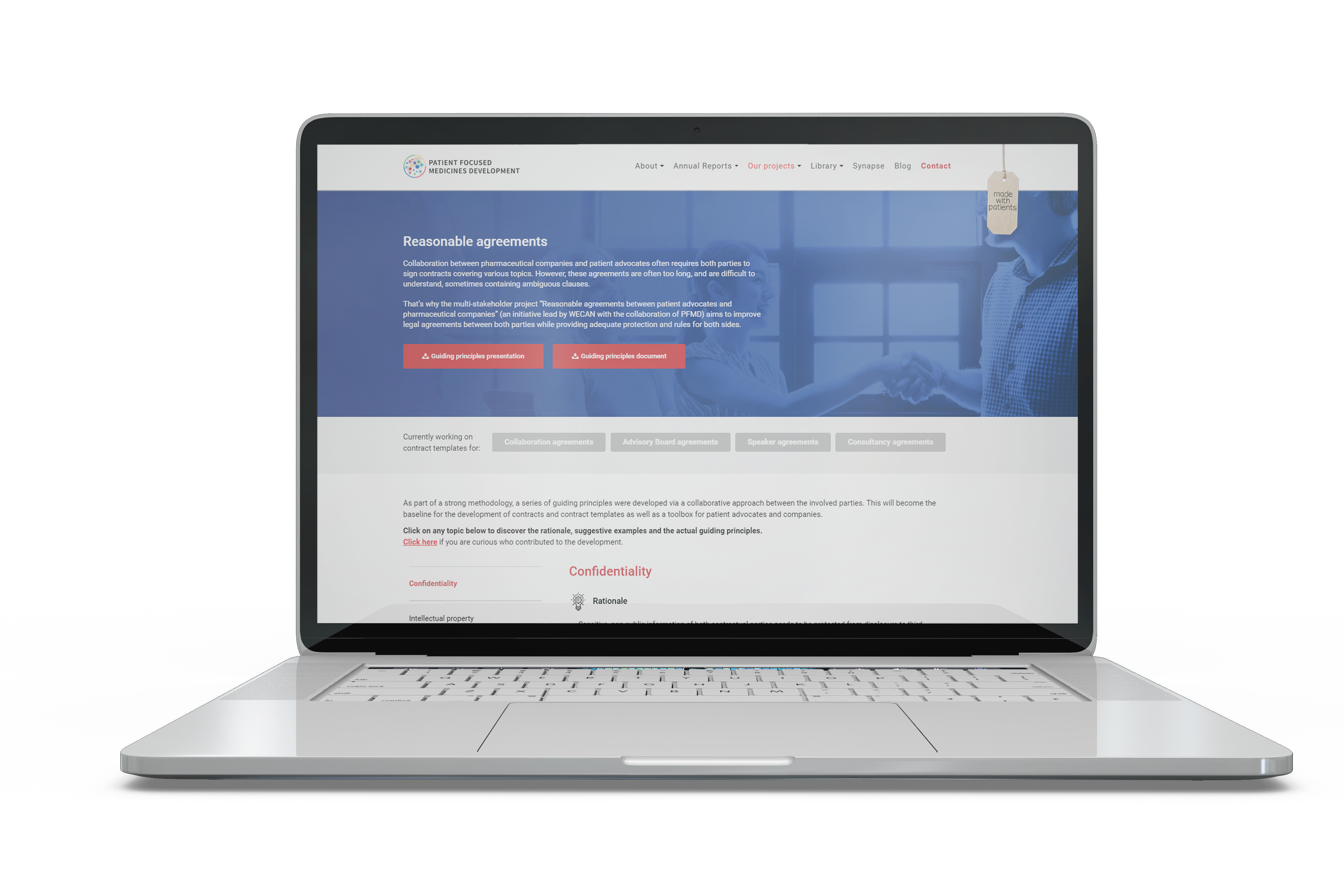
Read moreReasonable Legal Agreements
The project aims to improve legal agreements between both parties while providing adequate protection and rules for all sides.
Read more





















.jpg)