2021 Patient Focused Medicines Development Annual Report
A global collaborative non-competitive coalition to Improve global health by co-designing the future of healthcare for patients WITH patients
Intro
In 7 years of PFMD activity we observed a clear evolution in a positive direction - with regulators and payers shaping, improving and harmonizing their practices and expectations; with an active patient community that continues to strengthen and build expertize, capacity and resources to help shape new treatments, devices, digital health and health systems; and a growing interest from industry to invest and develop effective patient engagement practices.
At the same time, fragmentation, siloes and complexity of our health systems have grown so big that those same health systems are slow to regroup and recenter around patients, and ultimately missing its full potential as a system to deliver better patient outcomes faster, balanced and usable to all. In such a fast moving environment, where key stakeholders and organization have each their own path to better patient outcomes only collaboration and partnership allows an overall coherent and patient centered health systemThis collaboration has to happen in a safe, pre-competitive, learning and neutral context prone to universal solution discovery and development
2021 saw PFMD’s first year of the Patient Engagement 2025 Next Level Strategy implementation. We continue to work and develop the previously agreed strategic directions for development: Scaling Patient Engagement in Drug Development, Building the conditions and enablers in Patient Engagement and Building Patient Engagement in digital health and data. Moreover, PFMD continuously grows its membership,partnerships and engagement activities to help increase global contribution, outreach and adoption of patient engagement good practices in device and drug lifecycles, digital health and health systems.
In the paragraphs below you can find out more about PFMD’s expansion in new and exciting areas - such as addressing core issue regarding clinical trials, taking a stab at harmonizing patient experience, continuing to advance the Patient Engagement Management Suite with new and more specific tools for systematic patient engagement, starting to shape the patient engagement landscape in medtech, and increasing PFMD’s network and outreach through initiatives like the Patient Engagement Open Forum and more.
PFMD comprises 42 organizations, over 12,000 followers & contributors and a viewership of over 2,562,143. We would like to acknowledge and thank all individual patient engagement (PE) contributors and ambassadors, all connectors and champions of collaboration and collective action. We would particularly like to thank all those involved in the projects listed below. Thank you for all your hard work and diligent efforts, even in the face of the unknown.
As 2022 is just around the corner, we need to remember that only together can we continue to build on our growing momentum to take PE to yet another level in further strategic areas, ensuring that PE capability, capacity, learning and assessment are meaningful and qualitative, supporting systematic change in the entire health system.

Executive Director

2021 Achievements
- Scaling Patient Engagement Deeper
- Scaling Patient Engagement Wider
- Building conditions and enablers for patient engagement
- Building Patient Engagement in Digital Health and Data
- New additions to PEM Suite
- Building capacity through the Patient Engagement Training
- Mobilizing a co-created, agile Monitoring & Evaluation (M&E) metrics builder for better patient engagement intelligence
New additions to Patient Engagement Management Suite
The Patient Engagement Management Suite (PEM Suite) - your global hub for practical tools to plan, assess and execute any patient engagement initiative was significantly enriched in 2021.
New practical How-To Guides, have been finalized and added to the suite in 2021, driving the organizational adoption of patient engagement.
Pilots are now ongoing, with both EMD Serono and Ipsen utilizing the Guide for Patient Engagement in Early Discovery & Preclinical phases.
Following the completion of nearly 1400 surveys by patients and healthcare professionals across 5 continents, an infographic was developed to further disseminate and communicate this work.

new How To Guides
people engaged
inputs from 4 Public Consultations
people involved in development
downloads
pilots ongoing
Building capacity through the Patient Engagement Training
Our training offering reached the next level in 2021, achieving an increased impact in industry as thousands benefited from our online Patient Engagement training courses in 212 organizations spanning 89 countries.
The training potential for impact increased through an accreditation from ACMA (The Accreditation Council for Medical Affairs), and an increased accessibility due to translations in 6 languages - German, French, Italian, Spanish, and Portuguese.
have been accredited by the ACMA. An incentive for Board Certified Medical Affairs Specialist (BCMAS) and Medical Affairs Professionals.
reached through a dissemination partnership with Xpeer
enrolled from 89 countries
accessing the training
Mobilizing a co-created, agile Monitoring & Evaluation (M&E) metrics builder for better patient engagement intelligence
Appropriate Monitoring and Evaluation (M&E) tools and approaches are essential for integral impact of patient engagement. In 2021 a PEOF session was organized to build on the M&E framework started as part of the PARADIGM project. It enabled insight gathering to provide direction and shape to an operational and actionable M&E tool.
A new multi-stakeholder workstream was initiated, with 19 people, representing the patient community, industry and academia. They worked towards a simplified framework for M&E, encompassing PARADIGM identified criteria, as well as the Patient Engagement Quality Guidance dimensions. The goal: turning them into an easy-to-use and actionable digital tool, fully integrated into Synapse.
The group co-developed the first mock-ups for the tool, and layed out the plans for the beta testing coming in 2022.
Communication was also enhanced via a presentation made at the Patient Centricity & Collaboration conference on November 9. Underpinning this was the increasing recognition that stakeholders require additional support and resources to implement an organizational M&E strategy beyond a metrics builder - therefore, this will guide our work in this area during 2022.
PFMD was also approached by several organizations to collaborate on further developing a benchmarking tool for industry - this represents an exciting opportunity and leads to further consideration of organizational benchmarking and opportunities for PFMD to advance the development of a sector-wide rating/ranking system to further support organizational needs with regard to monitoring.
registered for the PEOF session on M&E
of the metric builder tested internally
involved in the multistakeholder workstream established
on new benchmarking tool
- Securing Patient Engagement in Patient Experience Data to drive momentum & better decision-making for all stakeholders
- PFMD adapted tools help to strengthen capabilities for further patient engagement in the MedTech sector
- Engaging in scalable capacity & capability programs for the acceleration of Patient Engagement in Asia
Securing Patient Engagement in Patient Experience Data to drive momentum & better decision-making for all stakeholders
Significant efforts have continued to fuse PE and patient experience data (PED), through our Patient Engagement & Patient Experience Data (PED) project.
370+ people got involved in the project development, through two dedicated sessions at the Patient Engagement Open Forum (PEOF) in 2021 and various working groups. A Steering Committee for the Collective Value Publication and Taxonomy workstreams was established. This multi-stakeholder, global group includes over 40 members representing industry, patient community, healthcare professionals, academia, regulatory bodies and HTA agencies.
Last year also saw the publication of our Highlighting Recent Trends in the Fast-Evolving Patient Engagement & Patient Experience Data Landscape report, which taught us that as patient engagement has become more prevalent in medicines development, the development and use of patient experience data is another critical way to further engage patients.
During 2021 IMI-PREFER and PFMD initiated a collaboration to support the IMI-PREFER sustainability goals and we remain in ongoing conversations to discuss patient preference studies, which are a valuable type of Patient Experience Data.

2021 also provided an opportunity for the project to become more closely aligned with the National Health Council Patient-Centred Core Impact Set (PC-CIS) initiative, involving active participation on their advisory council and workstreams to ensure ongoing alignment and a complementary perspective.
dynamic PEOF sessions with input from 370+ stakeholders. Check out the session recordings here and here
IMI-PREFER/PFMD collaboration
new steering committee of 40+ stakeholders established
people engaging with the PED outputs
of landmark report on trends in PE and PED. Download now
alignment with NHC PC-CIS initiative
PFMD adapted tools help to strengthen capabilities for further patient engagement in the MedTech sector
2021 saw a clear expansion of PFMD’s impact in the MedTech sector. A motivated and dedicated working group helped adapt some of our most-used tools to help strengthen capabilities for further patient engagement within the MedTech sector.
The Patient Engagement Quality Guidance, the Patient Engagement Digital Network, our Training Platform and our reference contracts were amended to reflect the nuances of this fast-growing sector that is increasingly interwoven with traditional healthcare.
We had a dedicated PEOF session for medtech and produced six editorial pieces on the hot topic available on PFMD’s news section.
Adapted Patient Engagement Quality Guidance for the medical device industry

Adapted Synapse Software to include medical device companies
Validation of the Training Platform’s Usefulness and Proposals for Future Updates

Adapted Reference Contract for collaborations with patients
PFMD MedTech member - Edwards LifeSciences
adapted tools completed, launched and integrated into PEM Suite
related trainings completed with 7 medtech companies
An inspiring PEOF session with 325 registration. Check out the recording here
from the medical device industry and patient organizations; working together to advance patient engagement
Engaging in scalable capacity & capability programs for the acceleration of Patient Engagement in Asia
This key area for PFMD was a busy one during 2021. PFMD actively contributed to HTA & regulatory dialogue in Asia, working with the Center of Regulatory Excellence (CoRE) and Coalition to Accelerate Patient Engagement in Asia-Pacific (CAPE).
The PFMD PE & PED project also delivered a presentation at the 2021 CAPE Regional Multi Stakeholder Roundtable: Embedding Patient Engagement for Sustainable Health Systems. 50 + participants representing regulatory and HTA Asian agencies were present.
In the background, dialog with regional partners was ongoing as we sought to develop and support capacity & capability-building programs, including the sharing and translation of PFMD PE resources

contribution to HTA dialogue in ASIA
with CoRE and CAPE
at 2021 CAPE Regional Multistakeholder Roundtable
with regional partners across Asia
- Forging the first large-scale co-creation conference in Patient Engagement Open Forum
- PFMD’s growing exposure and impact through the Patient Engagement community
- Embedding co-created and harmonized Global Principles and Methodology for patient engagement remuneration
- Growing the global patient engagement digital map & network on Synapse
- PFMD Membership Growth and growing collaborative network
Forging the first large-scale co-creation conference in Patient Engagement Open Forum
Building our capacity and capability was of course a priority once again last year. We poured our resources into our networks and tools that bring those with an interest in PE together.
For example, in 2021, the Patient Engagement Open Forum celebrated its first full and successful year beyond Paradigm. The event continued to prove its sustainability as a co-project organized by PFMD, EUPATI and European Patients’ Forum (EPF). Together, we have created a forum to shape and co-create real solutions FOR patients and WITH patients while providing a holistic perspective of patient engagement, its landscape and actors.



successful editions in 2021: April, June, October, December. Click here to watch session recordings of all 16 sessions
Geographical coverage
reached through communication activities
exceptional speakers
themese covered(digital health, patient experience data, precision medicine, patient engagement and medtech, fair remuneration for patients, etc)
registrations
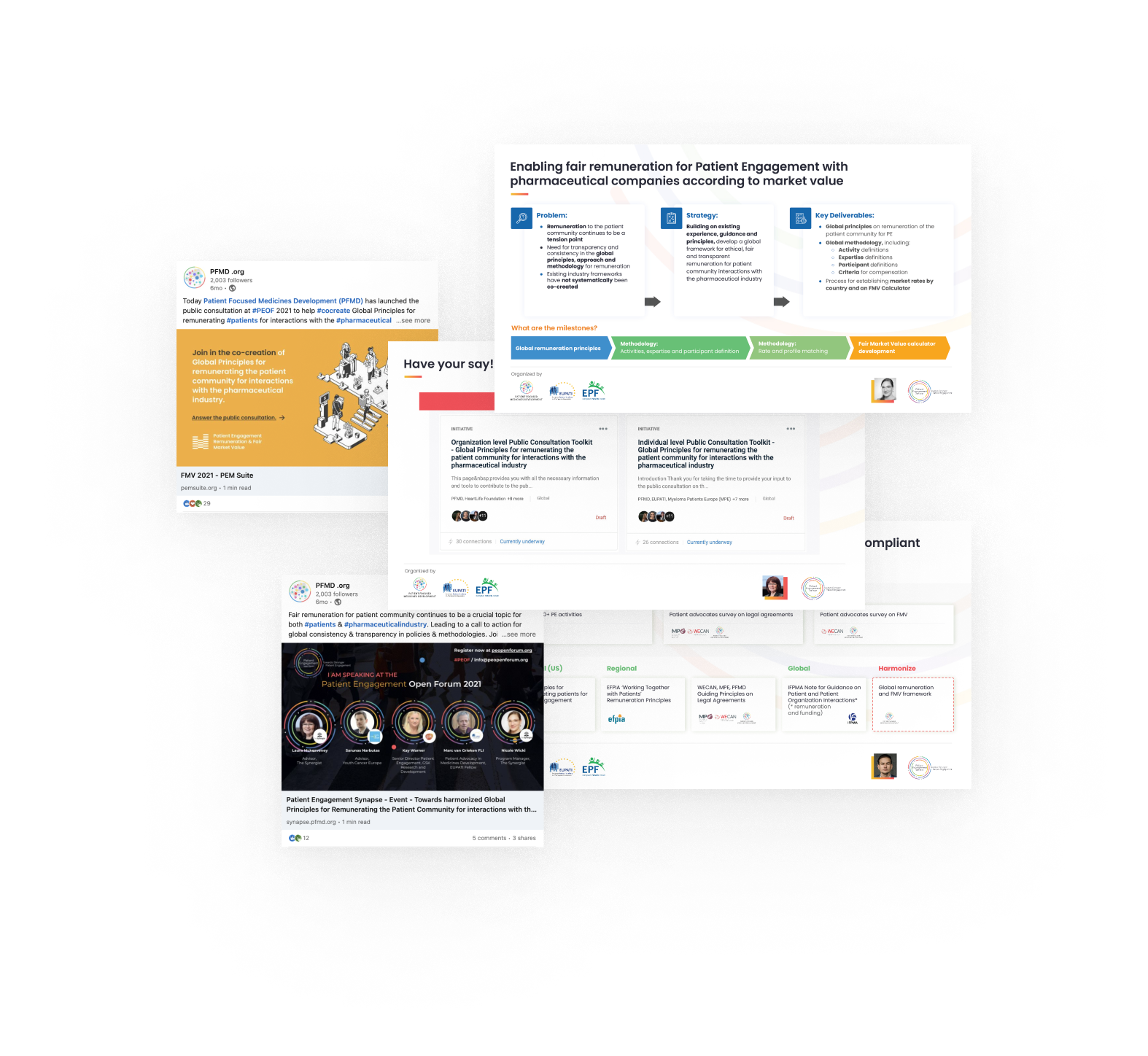
Embedding co-created and harmonized Global Principles and Methodology for patient engagement remuneration
The goal of this project is to establish a trusted process to remunerate the patient community for interacting with the pharmaceutical industry through patient engagement activities. Over 20 pharmaceutical companies and 40 patient organizations have been involved in the co-creation process until now, together with key bodies such as the IFPMA Patient Centricity Working Group, the Efpia Ethics and Compliance Committee, and the Efpia Patient Think Tank.
A dynamic and interactive PEOF session on the topic, with over 90 engaged participants, took place in October 2021. Check out the session recording here.
A set of Harmonized Global Principles for remunerating the patient community for interactions with the pharma industry were co-created. A public consultation was launched. It involved 200+ individuals, including 95 survey responses and more than 15 organizational feedback reports.
Ongoing efforts to disseminate the outputs of the co-creation activities included the publication of two articles on the PFMD blog. As we look towards 2022, the next steps are to bring forward international standards on patient community remuneration through the co-creation of a Global Frameworks and a Methodology for determining remuneration at market value.
with 20+ pharmaceutical companies and 40+ patient organizations
Public consultation launched to test the first Harmonized Global Principles. Download the Global Principles now
with key bodies including IFPMA and EFPIA
Interactive PEOF session with 90+ participants in October 2021
in patient remuneration in progress
PFMD’s growing exposure and impact through the Patient Engagement community
PFMD’s network and impact grows every year, due to the relentless support of its members and contributors, as well as their involvement in advancing patient engagement across many different dimensions.

Follow PFMD activity across all our communication channels and share relevant news and updates within your network
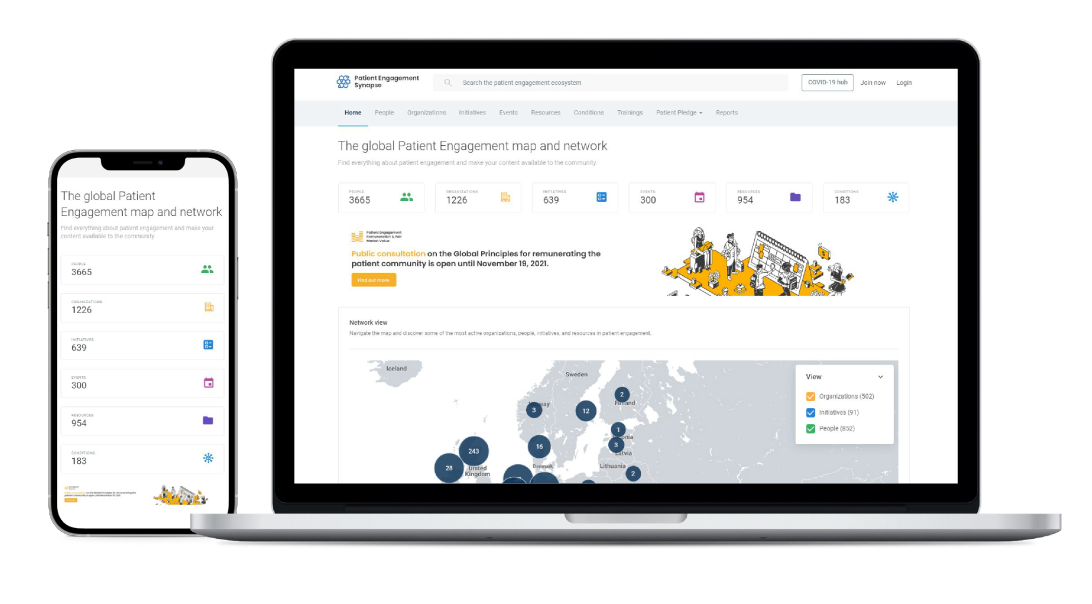
Growing the global patient engagement digital map & network on Synapse
Our increasing exposure and impact was also felt in the momentum driving the online Global Patient Engagement Network Synapse as significant growth took place in its digital map and network during 2021.
Synapse was subject to a series of enhancements that improved its usability and made it even more user-friendly. New developments meant that anyone can now use Synapse to organize and run registrations to their PE events.
Synapse accounts can now be used to access other health system lenses and networks, such as the Precision Medicine Synapse, Medical Technology Synapse, Digital Health Synapse and the Maternal Health Synapse.
Dynamic country pages have been introduced, meaning each country can now showcase its own initiatives, people and events.
Another level of security was also introduced for members, who can now log into Synapse using their own organization credentials (Single Sign On - SSO).
In addition, the conditions page usability was further improved, with an interactive map now available to show the precise location of conditions focus (see lung cancer condition page as an example).
PE people
Organizations
Initiatives
Resources
Events
Training courses in the Patient Engagement Training Catalogue
Over 10 development releases in 2021, meaning new features, enhancements and improvements
PFMD Membership Growth and growing collaborative network
New PFMD members in 2021
PFMD continues to welcome new members from across the entire PE ecosystem as we enrich and broaden our membership base year on year. New members that joined our ranks in 2021 include Sanofi, Global Heart Hub, Digestive Cancers Europe, Lupus Foundation of America, European AIDS Treatment Group, Edwards Life Sciences, and Syndrome de Dravet Fundacion.
New and deepening relationships with key players in the health ecosystem
We also continue to build new and deepening relationships with key players in the broader health ecosystem, such as IMI, the FDA, EMA, HTAi, ISPOR, IFPMA, Bio and ARCS.
- Clinical Trial Information Network: connecting information providers with information users with new distribution capacity
- Establishing a Patient Engagement Framework for Digital Health
Clinical Trial Information Network: connecting information providers with information users with new distribution capacity
The Clinical Trial Information Network Project addresses core issues within clinical trial information sharing and aims to empower all potential beneficiaries with a view to better performance and ultimately better health outcomes.
By creating a new distribution capacity to optimize the exchange of standardized clinical trial information, the project’s goal is to improve patient matching to clinical trials and enable all players to further innovate.
The focus during 2021 was to create a solid foundation for the project by bringing together the right stakeholders and mix of expertise with whom to analyze the current ecosystem in order to draft a sustainable concept and plan. This was achieved by landscape mapping of the current clinical trial information ecosystem and in-depth interviews with both patient organizations and pharma collaborators on existing challenges and bottlenecks.
This allowed us to identify existing gaps and opportunities for collaboration, helping shape the Project Roadmap and incremental project phases, as well as the Proof of Concept Pilot. As a result, new multi-stakeholder collaborations were established with Codex, Trialscope, Breast Cancer Trials and Efpia.
.png)
carried out around existing initiatives, and including reports from ACS-CAN, Transcelerate, CISCRP, Lung Cancer Europe and others.
In-depth interviews with patient groups and industry to assess needs
finalized as project progressed
collaborations established to broaden impact, including 6+ patient advocates, 5+ industry representatives, and more than 4 clinical trial standards and data experts.
Establishing a Patient Engagement Framework for Digital Health
2021 marked a major push in our dedicated efforts to map, understand, define and align on the role of patients in digital health. To this end, we identified key issues and goals for 2021 and formed a project roadmap with key digital actors.
A strong project foundation was established through multi-stakeholder interviews, including digital champions from organizations such as the FDA, The Genetic Alliance, EURORDIS, EUPATI, Data Saves Lives, Roche, Takeda, Janssen & Patvocates. With their support and through rigorous desk research, we carried out a major landscape analysis and gap identification of existing data management guidelines, good practices, and patient expectations.
Over 850 participants were engaged to co-create and design the patient needs and priorities of the digital project through three live Patient Engagement Open Forum sessions:
As a result of our efforts three distinct work streams were formed:
through 3 PEOF sessions with 850+ participants
formed that focuses on 3 major workstreams
a multi-stakeholder digital champions team
literature and landscape review to map and analyze the digital patient engagement ecosystem, including over 100 initiatives and resources and 20+ interviews
a digital toolkit that will become publicly available in the summer of 2022
Financial Report
The Synergist goal is to bring all relevant stakeholders around the table to work together for societal impact. In a world of scarce resources, we expect the for-profit side to support societal impact and enable the participation of not-for-profit contributors. The Synergist, as a not-for-profit, is housing these afore-mentioned programs. They are managed through comprehensive governance structures, but not as separate legal entities. Each program benefits from full financial autonomy, including audit.
 Turnover
Turnover
2.067.956
 Project Expenses
Project Expenses
2.008.346
 In-kind Hours
In-kind Hours
3453.49
 In-kind Contribution
In-kind Contribution
278.006
Split of PFMD Turnover
 Membership fees
Membership fees
1.400.000
 Specifically funded projects
Specifically funded projects
534.998
 Other
Other
132.958
The PFMD Board
The PFMD Board Members consists of:

Kelli Collins
Vice President Patient Engagement, National Kidney Foundation

Esther Krofah
Executive Director, FasterCures (A Center Of The Milken Institute)

Anthony Yanni
SVP And Global Head, Patient Centricity, Astellas Pharma US

Andrew Benzie
Head of Patients in Partnership Programme, GSK

Jim Elliott
Public Involvement Lead, Health Research Authority

Sanja Njegic
Global Head Of Patient Partnership, Roche

Graeme Johnston
Patient Advocate, Individual/ Patient

Lode Dewulf
Chief Patient Officer At Servier Group

Begoña Nafría Escalera
Patient Engagement In Research Coordinator- Hospital Sant Joan De Déu

Neil Bertelsen
Chair, HTAi Patient And Citizen Involvement Interest Group

Jan Geissler
Patient Advocate, EUPATI

Tony Hoos
Integral Health

Eleanor M. Perfetto
PhD, MS, Interim CEO and EVP, National Health Council

Geraldine Reillyo
Global Patient Engagement lead, Gilead Sciences

Marc Boutin
Chief Executive Officer, National Health Council

Alexandra Moutet
Global Head of Patient Affairs, UCB

Costin (Radu) Ganescu
Vice President and Treasurer, European Patient Forum (EPF)

Leanne West
President, International Children’s Advisory Network (iCAN)
The Team

Helena Harnik
Synergist Programs Director

Quentin Clermont
Chief Operations Officer

Nicole Wicki
Program Manager

Jean-Christophe Capelle
Financial Director

Chi Pakarinen
Program Manager

Roxana Radu
Communication Manager

Matt Szafert
Executive Assistant

Lise Brooke
Growth Hacker

Gulwish Ahmed
Communications Coordinator

Laila Deeb
Web Developer

Maximiliane Rauch
Senior Project Coordinator

Anne-Marie Hamoir
Senior Consultant

Ify Sargeant
Scientific Editor

Jeremy Jamar
IT Architect

Daniela Luzuriaga
Senior Project Coordinator

Carina Prelucan
Project Coordinator

Elizabeth Priest
Program Manager

Hayley Chapman
Program Manager

Silvia Bornengo
Senior Project Coordinator

Diana Aron
Senior Project Coordinator

Maddalena Benivento
Project Coordinator

Danielle Barron
Editor in Chief

Gary Finnegan
Editor in Chief